Quick Links
A healthy immune system is essential for a superior quality of life. Evolution has allowed the immune system to develop complex strategies to protect us from many life-threatening diseases and infections. However, the immune system can become genetically altered by exposure to constantly changing environments, resulting in dramatic consequences for the immune system and overall health. Alterations can result in different diseases, including autoimmune diseases, allergies, immunodeficiencies, and cancer. Dr. Elkhal’s research program investigates these alterations that target the adaptive and/or innate immune system and aims to re-establish an adequately balanced immune response. His lab uses a combination of animal disease models, genetics, cell biology, immunology, biochemistry, and imaging techniques to achieve their research goals.
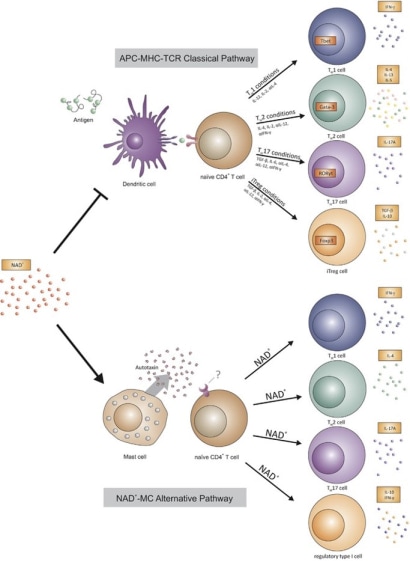
For half a century, scientists have made tremendous progress in understanding how the immune system communicates and provides protection. It is now well established that the adaptive immune response requires the entry of a pathogen (i.e., bacteria, virus) to trigger our immune response. This pathogen is captured by innate immune cells, termed antigen-presenting cells (APCs), that process the antigen into peptides and present it at their surface via the MHC class molecule (MHC I or II) to a T cell receptor (TCR) of a lymphocyte (CD4+ or CD8+ T cells). These discoveries enable a better understanding of how immune cells communicate to protect against infection, leading to the development of vaccines.
Currently, novel therapies are based on this model to develop chimeric antigen receptor-modified T (CAR-T) cell therapies to treat some blood cancers (B cell-lineage lymphoma and leukemia). Although these medical breakthroughs lead to the development of new therapies, alterations that target this pathway have been a limiting factor. Some patients exhibit an alteration within the MHC or TCR molecules, affecting APC-T cell communication that is either altered or absent. Patients with primary immunodeficiencies (PIDs) are highly susceptible to infection and cannot mount a proper immune response to eliminate the pathogen. Moreover, an exaggerated T-cell response has been shown to result in autoimmune diseases or atopic disorders (Fig. 1).
The immune system's role is increasingly recognized as a contributing factor in neurological disorders such as Alzheimer's disease, epilepsy, Parkinson's disease, and cardiovascular diseases. In Alzheimer's disease, persistent neuroinflammation resulting from immune system activation is believed to contribute to the progressive neurodegeneration observed in affected individuals. Epilepsy is linked to an increased immune response in the brain, potentially leading to seizures and cognitive deficits. In the case of Parkinson's disease, neuroinflammatory processes are evident, with microglia and astrocytes, key immune cells in the central nervous system, becoming overactive and potentially contributing to the death of dopamine-producing neurons.
The immune system plays an essential, multifaceted role in cardiovascular diseases, especially in conditions like cardiac hypertrophy and myocardial infarction. In cardiac hypertrophy, a condition marked by the thickening of the heart muscle, the immune system is central to both initiating and resolving the pathological process. When the heart is exposed to various stressors, immune cells are recruited to the heart, leading to inflammation and fibrosis. Prolonged inflammation can worsen hypertrophy, ultimately contributing to heart failure.
Conversely, in myocardial infarction, the immune system's response is crucial in clearing cellular debris, facilitating tissue repair, and forming scar tissue. However, an excessive or prolonged immune response can result in unfavorable cardiac remodeling and impaired function. Therefore, comprehending the immune system's involvement in neurologic and cardiomyopathic conditions is vital for developing innovative therapeutic strategies aimed at mitigating inflammation and supporting tissue repair, ultimately enhancing the treatment of neurological disorders and cardiomyopathies.
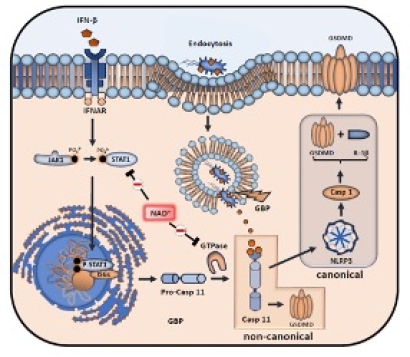
Dr. Elkhal’s laboratory has discovered that nicotinamide adenine dinucleotide (NAD+) can elicit an immune response independently of antigens, antigen-presenting cells (APCs), and MHC-TCR interactions. Their research revealed that NAD+ activates this response through mast cells, which are traditionally associated with allergic immune reactions. Notably, their work was the first to identify NAD+ as a key regulator of adaptive immunity, highlighting its strong immunosuppressive effects in pre-clinical models of autoimmune diseases such as multiple sclerosis and transplant rejection. Beyond its immunosuppressive role, Dr. Elkhal's team has shown that NAD+ administration provides protection against infections and prevents death from septic shock by targeting the non-canonical inflammasome pathways of macrophages (Fig.2). While these findings introduce a novel mechanism influencing both innate and adaptive immunity, the intricate processes through which NAD+ mediates immunosuppression and simultaneously defends against infections and sepsis-induced mortality remain to be fully understood.
The lab is still investigating how NAD+ regulates other immune cells and its application in other life-threatening diseases. Dr. Elkhal's pioneering work has elucidated a novel immune pathway that does not require traditional immune components like APCs and MHC-TCR interactions. This pathway exhibits the capability to modulate an array of immune cells, encompassing macrophages, dendritic cells, mast cells, CD4+ and CD8+ T cells. This exciting discovery is poised to enhance our comprehension of immune cell communication and offers promising prospects for the development of innovative therapeutic approaches for conditions such as autoimmune diseases, hemophilia, neurological disorders, and cardiomyopathies.
Fig. 1. Novel mechanism of T-cell differentiation mediated by NAD+
Fig. 2. Macrophage-pyroptosis inhibition mediated by NAD+.